Biologics
A guide to biologics & alpha-gal syndrome (AGS)
AGS and biologics
Select publications
on AGS and biologics
Cell lines
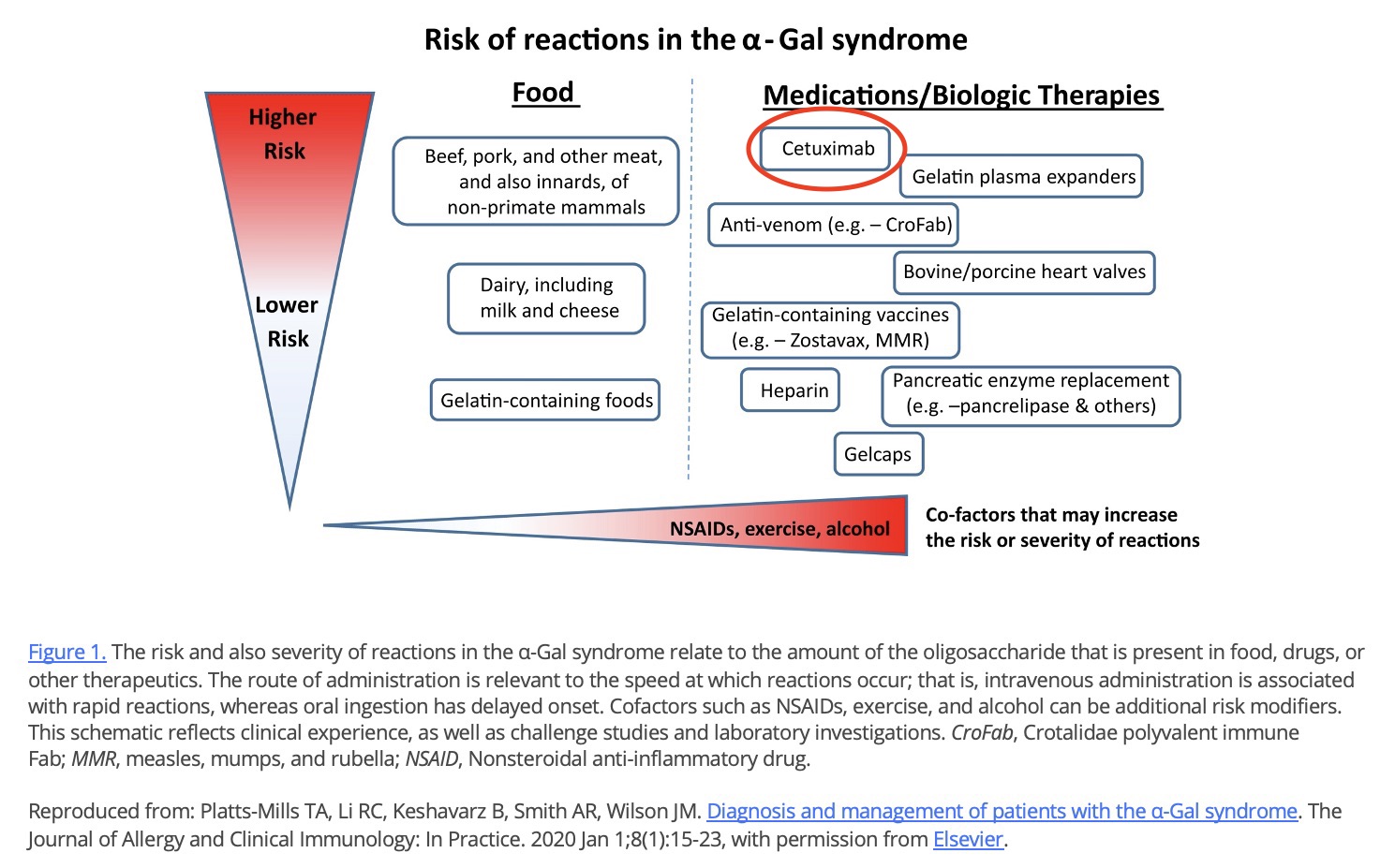
Cetuximab
Ustekinumab
Infliximab
Abatacept
Alpha-gal syndrome and biologics
Engineered antibodies: Many monoclonal antibodies (mAbs) used clinically are produced in non-primate mammalian cell lines; however, the evidence for alpha-gal expression on most mAbs other than cetuximab is minimal. One exception is abatacept (Orencia), which is manufactured in a Chinese hamster ovary (CHO) cell line and also shown to contain the alpha-gal epitope. Three of our patients reported reactions to abatacept, and we know of a colleague, Dr. David Fitzhugh (Chapel Hill, NC), who has seen a patient with AGS that had ‘immediate and profound anaphylaxis’ to IV abatacept (personal communication). Another exception is infliximab, which has been shown to express low amounts of alpha-gal and, despite wide usage, has been linked to only a small number of reactions in patients with AGS. Despite an apparent risk with some CHO cell-produced mAbs, we have successfully administered omalizumab to numerous patients with AGS. Perhaps the route of delivery is critical to creating a risk for reaction; nevertheless, we proceed with caution (e.g., divided dose 10-90 plus extended observation time) on first dose of any mAb produced in a mammalian cell line.
Monoclonal antibodies: Despite the fact that many mAb that are in clinical use are generated in non-primate mammalian cell systems, the evidence for α-Gal expression on most monoclonals other than cetuximab is minimal. One exception is infliximab, which has been shown to express low amounts of the glycan and has been linked to reactions in a small number of subjects with the α-Gal syndrome. The reason why α-Gal glycosylation of the Fab occurs selectively on cetuximab as compared to other monoclonal is likely due to differences in the cell lines used in production. Cetuximab is produced in mouse SP2/0 cells whereas most other monoclonals are generated in other cell lines, such as Chinese Hamster Ovary cells. The specific amino acid sequence in the Fab of cetuximab could also play a role in favoring glycosylation.
The majority of biologics are made in non-primate mammalian cell lines, mostly Chinese hamster ovary (CHO) or murine (NS0 or Sp2/0) cells (cit#). Murine cells, and to a lesser extent CHO cells, can incorporate alpha-gal into their glycoproteins and glycolipids (cit#).
- Some biologics made in mammalian cell lines are glycosylated with alpha-gal (6,57).
- The most notorious of these is cetuximab, which can cause severe reactions in people with AGS and has been associated with more than ten deaths (6,30,31,57,86, cit#).
- Some biologics contain alpha-gal but do not bind patient IgE in vitro, possibly due to the configuration of alpha-gal or other factors (cit#).
- For other biologics, there is no evidence of glycosylation with alpha-gal (6,57).
- Manufacturers monitor glycosylation of biologics, but they do not release this information to the public. This leaves both providers and patients guessing as to whether individual products are glycosylated with alpha-gal or not.
Factors that may influence the likelihood that individual biologics made in mammalian cell lines will cause reactions in patients with AGS include (cit#):
- The cell line (NS0 vs Sp2/0 vs CHO) used in production
- The number of alpha-gal sugars attached
- The configuration of alpha-gal
Biologics for which there are published reports of hypersensitivity reactions in patients with AGS include:
- Cetuximab (severe reactions, at least eight deaths) (6,30,31,57,86, cit#)
- Abatacept (Orencia) (cit#,57)
- Infliximab (Remicade) (6,57,67)
- Ustekinumab (Stelara) (cit#, cit#)
Evidence for alpha-gal expression on most other mAbs is minimal (6,57), but expert opinion is to proceed with caution on first dose of any mAb produced in a mammalian cell line (57).
Select publications
on AGS and biologics
Select publications on AGS and biologics
General:
- Hatfield G, Tepliakova L, Tran J, Lu H, Gilbert M, Tam RY. Bivalent non-human gal-α1-3-gal glycan epitopes in the Fc region of a monoclonal antibody model can be recognized by anti-Gal-α1-3-Gal IgE antibodies. MAbs. 2023;15(1):2239405.
- Hinterholzer A, Moises J, Regl C, et al. Unambiguous identification of α-Gal epitopes in intact monoclonal antibodies by NMR spectroscopy. MAbs. 2022;14(1):2132977.
- Mangla A, Agarwal N. Relevance of Anti-Galactose-α-1,3-Galactose Antibodies in the Era of Monoclonal Antibodies. J Oncol Pract. 2019;15(12):679-680.
- van Bueren JJL, Rispens T, Verploegen S, et al. Anti-galactose-α-1,3-galactose IgE from allergic patients does not bind α-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29(7):574-576.
- Bosques CJ, Collins BE, Meador JW 3rd, et al. Chinese hamster ovary cells can produce galactose-α-1,3-galactose antigens on proteins. Nat Biotechnol. 2010;28(11):1153-1156.
- Pointreau Y, Commins SP, Calais G, Watier H, Platts-Mills TAE. Fatal infusion reactions to cetuximab: role of immunoglobulin e-mediated anaphylaxis. J Clin Oncol. 2012;30(3):334; author reply 335.
- Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IGE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109-1117.
Expert opinion:
- Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16(7):667-677.
- Platts-Mills TAE, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and Management of Patients with the α-Gal Syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15-23.e1.
Olaratumab: (removed from market)
- Van Tine BA, Govindarajan R, Attia S, et al. Incidence and Management of Olaratumab Infusion-Related Reactions. J Oncol Pract. 2019;15(11):e925-e933.
Cell lines
Chimeric mouse-human monoclonal antibodies, notably cetuximab, contain alpha-gal glycosylation in the Fab and Fc domains and severe hypersensitivity reactions to cetuximab have been associated with IgE antibodies against alpha-gal. Like cetuximab, infliximab and ustekinumab are expressed in the murine mouse myeloma sp2/0 cell line, allowing for the alpha-gal glycosylation, though only in the Fc domain.
For years it was thought that CHO cells lack the machinery to synthesize α-Gal and therefore represent a more suitable production system for protein therapeutics, although the ability of CHO cells to produce the α-Gal epitope in recombinant proteins was previously reported5. Recently, Bosques et al.6 have identified a functional CHO ortholog of N-acetyllactosaminide-3-αgalactosyltransferase-1, showing that the therapeutic product abatacept (Orencia, CTLA4-IgG fusion protein), produced in CHO, contains α-Gal residues.
Taken together, these results show that commercial biotherapeutics manufactured in CHO can, in fact, contain α-Gal and suggest that the enzyme product of the identified CHO Ggta1 has the appropriate activity to produce α-Gal–containing products.
Chinese Hamster Ovary (CHO) cells
The majority of biologics are made in Chinese hamster ovary (CHO) cells. Previously, it was believed that CHO cells lacked the machinery to synthesize alpha-gal, but more recent research shows that CHO cells can, at times, glycosylate with alpha-gal (cit#,57).
With the exception of abatacept (Orencia), there is a lack of evidence for glycosylation with alpha-gal or associated reactions in products made in a CHO cell line (6,57). However, experts recommend caution on first dose of any mAb produced in a mammalian cell line (57).
NS0 and Sp2/0 cells
Biologics made in NS0 and Sp2/0 cells are more likely to be glycosylated with alpha-gal than products made in CHO cells (cit#).
Cetuximab (Erbitux)
Cetuximab is a cancer drug made in Sp2/0 cells. It played a role in the discovery of AGS (6,30,31,57). It is the single, riskiest known medical product for people with alpha-gal syndrome. Cetuximab can cause severe reactions and has been associated with more than ten fatal, alpha-gal-related reactions (86, cit#).
There is a growing body of research on cetuximab and AGS, including a desensitization protocol (see below).
Select publications of AGS and cetuximab
There are quite a few papers on cetuximab and AGS. See the AGI Publications database for more.
- Della Marta N, Yuile A, Mok C, et al. A protocol for pretreatment testing for antibodies to galactose-alpha-1,3-galactose to mitigate the risk of cetuximab hypersensitivity reactions. J Clin Oncol. 2025;43(4_suppl):115-115.
- Kopač P, Koren A, Bidovec-Stojkovič U, et al. Basophil activation test predicts cetuximab anaphylaxis severity in alpha-gal IgE-positive patients. Diagnostics (Basel). 2024;14(13):1403.
- Popa CM, Cherciu Harbiyeli IF, Ciurea AM, et al. A preliminary study examining the correlation between EGFRI treatment, clinic dermatoscopy features, and serum levels of anti-alpha-galactosyl IgE in colorectal cancer patients. Gastroenterol Insights. 2024;15(2):505-518.
- Li S, Zhang Y, Zha J, Chen W. Successful administration of cetuximab using dose escalation method: a case report. J Med Case Rep. 2024;18(1):479.
- Wen S, Unuma K, Chinuki Y, Hikino H, Uemura K. Fatal anaphylaxis due to alpha-gal syndrome after initial cetuximab administration: the first forensic case report. Leg Med. 2021;51:101878.
- Park KH, Lee J, Beom SH, et al. Nationwide pharmacovigilance data for cetuximab-induced anaphylaxis and predictive model validation using prospective specific IgE detection. World Allergy Organ J. 2021;14(7):100553.
- Li Jennifer Chen, LaHood Nicole A., O’Donnell Paul V., Banerji Aleena. A 67-Year-Old Man with Pruritus and Dyspnea. NEJM Evidence. 2022;1(11):EVIDmr2200210.
- Yuile A, Fanuli C, van Nunen S, et al. Increased rates of cetuximab reactions in tick prevalent regions and a proposed protocol for risk mitigation. Asia Pac J Clin Oncol. Published online September 24, 2020. doi:10.1111/ajco.13465
- Atwal D, Safar AM, Govindarajan R, Makhoul I. Severe first infusion reaction related to cetuximab in cancer patients in Arkansas. J Oncol Pharm Pract. 2019;25(5):1130-1134.
- Serrier J, Davy JB, Dupont B, et al. Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab. BMC Cancer. 2023;23(1):32.
- Pointreau Y, Commins SP, Calais G, Watier H, Platts-Mills TAE. Fatal infusion reactions to cetuximab: role of immunoglobulin e-mediated anaphylaxis. J Clin Oncol. 2012;30(3):334; author reply 335
- Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IGE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109-1117.
Ustekinumab (Stelara)
We posit that our patient’s anaphylaxis was due to IgE-mediated hypersensitivity reaction against alpha gal present on ustekinumab, a fully humanized monoclonal antibody expressed through the same murine cell line as cetuximab and infliximab.
In this study, patients with the alpha-gal mammalian meat allergy exhibited IgE binding to ustekinumab, but not to a representative mAb generated in CHO cells. The level of IgE binding to ustekinumab was lower than that for cetuximab, but still detectable in the assay. The difference may be due to the established presence of α-gal on the Fab portion of cetuximab, whereas for ustekinumab it is reportedly limited to the Fc domain. When administered subcutaneously, ustekinumab was successfully tolerated by all of the patients who had reacted to the initial intravenous infusion. Additional studies evaluating the degree of IgE binding and basophil activation with exposure to various mAb concentrations would help determine if a dose-response relationship exists and could aid clinicians in optimizing the safe use of these monoclonal antibodies for patients with α-gal syndrome. For patients with mild to moderate hypersensitivity reactions to intravenous ustekinumab, switching to subcutaneous ustekinumab and considering referral to an allergist for monitored administration is advisable. In our experience, pre-medication before subcutaneous injections is reasonable but not required.
Select publications on ustekinumab and AGS
- Ritaccio G, Muratore A, Kakadiya P, McGill S. S109 itching for therapy: Alpha-gal syndrome as a cause of anaphylaxis to ustekinumab. Am J Gastroenterol. 2024;119(12S):S29-S29.
- Venkat P, Nehrbas J, Figueroa E, Behm B, Wilson J. S39 first-dose infusion reaction to ustekinumab is associated with the presence of serum IgE against galactose-α-1,3-galactose (α-gal). Am J Gastroenterol. 2024;119(12S):S11-S11.
- Blauvelt A, Papp K, Trivedi M, et al. Efficacy and safety of the ustekinumab biosimilar ABP 654 in patients with moderate-to-severe plaque psoriasis: a randomized, double-blinded, active-controlled, comparative clinical study over 52 weeks. Br J Dermatol. Published online October 23, 2024:ljae402.
Infliximab (Remicaid)
We describe a pediatric patient with Crohn’s disease who developed urticaria and pruritus roughly 6 hours after her very first infliximab infusion that progressed to chronic urticaria following subsequent infliximab infusions. She was diagnosed with mammalian meat allergy based on an elevated serum IgE level directed against alpha-gal. Her symptoms resolved once infliximab infusions were discontinued and did not recur after commencing therapy with adalimumab.
Select publications on infliximab and AGS
- González Polanco E, Borowitz S. Delayed Hypersensitivity Reaction to Infliximab Due to Mammalian Meat Allergy. JPGN Reports. 2023;4(3):e322
- Chitnavis M, Stein DJ, Commins S, Schuyler AJ, Behm B. First-dose anaphylaxis to infliximab: a case of mammalian meat allergy. J Allergy Clin Immunol Pract. 2017;5(5):1425-1426.
- Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16(7):667-677.
- Platts-Mills TAE, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and Management of Patients with the α-Gal Syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15-23.e1.
Abatacept
In this study, patients with the alpha-gal mammalian meat allergy exhibited IgE binding to ustekinumab, but not to a representative mAb generated in CHO cells. The level of IgE binding to ustekinumab was lower than that for cetuximab, but still detectable in the assay. The difference may be due to the established presence of α-gal on the Fab portion of cetuximab, whereas for ustekinumab it is reportedly limited to the Fc domain. When administered subcutaneously, ustekinumab was successfully tolerated by all of the patients who had reacted to the initial intravenous infusion. Additional studies evaluating the degree of IgE binding and basophil activation with exposure to various mAb concentrations would help determine if a dose-response relationship exists and could aid clinicians in optimizing the safe use of these monoclonal antibodies for patients with α-gal syndrome. For patients with mild to moderate hypersensitivity reactions to intravenous ustekinumab, switching to subcutaneous ustekinumab and considering referral to an allergist for monitored administration is advisable. In our experience, pre-medication before subcutaneous injections is reasonable but not required.
Abatacept is used to treat autoimmune disease like arthritis. It is made in a CHO line. Unlike most biologics made in CHO cells, abatacept is glycosylated with alpha-gal and has been associated with reactions in some patients with AGS (cit#,57). See papers below for information about alpha-gal in abatacept and associated reactions.
Select publications on abatacept and AGS
- Bosques CJ, Collins BE, Meador JW 3rd, et al. Chinese hamster ovary cells can produce galactose-α-1,3-galactose antigens on proteins. Nat Biotechnol. 2010;28(11):1153-1156.
- Hinterholzer A, Moises J, Regl C, et al. Unambiguous identification of α-Gal epitopes in intact monoclonal antibodies by NMR spectroscopy. MAbs. 2022;14(1):2132977.
- Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16(7):667-677.