Dentistry
A guide to dentistry and alpha-gal syndrome (AGS)
Dentistry and alpha-gal syndrome
Some products used in dentistry contain mammal-derived ingredients or carrageenan. Dentists may need to modify the products they use during dental procedures for their patients with AGS.
To check whether products contain mammal-derived ingredients or carrageenan, dentists can:
- Contact the manufacturers or
- Ask Pill Clarity (formerly VeganMed) for assistance.
Highlights from
Alpha-gal syndrome: potential for a hypersensitivity reaction after the use of dental products
Literature review and case report
Brooks JK, Hoch NI, Hoch ER, Sultan AS.
May 15, 2025
Objective
” To review the general and pathophysiological features of AGS, increase awareness of its potentially serious immunologic consequences, and provide a list of dental products that pose an increased risk of causing an allergic reaction from cross-reactivity. In addition, a summary of published accounts of cases of AGS associated with use of dental products, including a clinical narrative of an affected patient, are presented.”
Methods
“An electronic search was conducted of PubMed and Google Scholar electronic databases for articles pertaining to AGS that appeared in the English-language literature from January 1, 1990, through November 30, 2024, including clinical investigations, review articles, case reports, and case series.”
Results
-
A review of dental products resulted in identificaiton of a number of excipients and products containing expressing alpha-gal or alpha-gal-like epitopes, see chart below.
-
The literature identified 4 cases of AG-based reactions linked to dental products, and with the addition of the authors’ case, 5 patients with AGS have documented sequelae associated with dental product use; 4 patients were exposed to animal-based hemostatic agents, and 1 patient reacted to doxycycline contained in animal-based gelatin capsules.
Highlights from
The Implications of Alpha-Gal Syndrome for Dental and Medical Treatment
Adam DeGenova MBA and Kristi Soileau
2025
Key Points
- “In a patient diagnosed with alpha-gal syndrome, until testing has proven otherwise, the dentist should assume that the sensitivity is significant, and thus should consider modifying dental treatment, being aware of all products placed into or onto any patient with AGS.”
- A number of products that may potentially contain alpha-gal were identified (see below).
- “It would seem wise to be on the lookout for those patients who may have an unusual possibly delayed immunological response following dental treatment, yet who have not to date revealed any diagnosis of AGS.”
Dental products and their excipients that could potentially trigger AGS reactions–identified by Brooks et al
Adapted from Brooks JK, Hoch NI, Hoch ER, Sultan AS. Alpha-gal syndrome: potential for a hypersensitivity reaction after the use of dental products: Literature review and case report associated with oral health care. The Journal of the American Dental Association. 2025 May 15.
| DENTAL PRODUCTS | EXCIPIENTS |
|---|---|
| Artificial saliva substitutes | Arachidyl propionate |
| Bone graft regenerative materials | Casein phosphopeptide–amorphous calcium phosphate |
| Chlorhexidine gluconate mouthrinses | Carrageenan |
| Collagen dermal fillers and plugs | Collagen |
| Cough suppressants | Gelatin |
| Fluoride mouthrinses or gels | Glycerin |
| Gelatin capsules | Glycerol |
| Gingival graft substitutes | Hydrogels |
| Hydrogels | Lactic acid |
| Impression compounds | Lactose monohydrate |
| Intraarticular joint lubricating solutions | Lanolin |
| Lip balms | Magnesium stearate |
| Mouthrinses | Milk proteins |
| Oral debriding agents | Oleic acid |
| Pharmaceutical patches | Stearate |
| Prophylaxis pastes | Stearic acid |
| Soft-tissue scaffolds | |
| Sore throat sprays | |
| Suture materials (eg, plain gut, chromic, and catgut) | |
| Toothpastes | |
| Tooth-whitening gels | |
| Vascular grafts, meshes, and patches |
Excerpts from Brooks JK, Hoch NI, Hoch ER, Sultan AS. Alpha-gal syndrome: potential for a hypersensitivity reaction after the use of dental products: Literature review and case report associated with oral health care. The Journal of the American Dental Association. 2025 May 15:
Gelatin
Gelatin is usually derived from hydrolyzed bovine and porcine collagen and is often included in a variety of dental products, such as hemostatic agents,18,34-44 pharmaceutical capsules, cutaneous patches, vascular materials (eg, meshes, patches, and grafts), bone graft regenerative materials, soft-tissue scaffolds, sutures (eg, plain gut, chromic, and catgut), collagen dermal fillers and plugs, osteochondral grafts, and viscosupplementation agents (intraarticular joint lubricating solutions).28-30,45-47
Glycerin
Glycerin, also referred to as glycerol, is processed from beef, sheep, and plant oils or fats and may be an excipient in toothpastes, mouthrinses, prophylaxis pastes, oral moisturizer preparations, endodontic debriding agents, chlorhexidine gluconate oral rinse, fluoride rinse or gels, gel capsules, artificial saliva substitutes, sore throat sprays, topical anesthetics, tooth-whitening gels, povidone-iodine solution, cough syrups, and electronic cigarette vaping liquids.48-50 Some antihistamine formulations contain animal-derived glycerin that could exacerbate an allergic reaction in susceptible people.
Hydrogels
Hydrogels are a group of complex polymers derived from mammalian sources (eg, human, bovine, and porcine) that potentially could contain AG epitopes, such as collagen, glycerin, albumin, placental tissue, and gonadotropin-releasing hormone.51 A broad range of dental products contain hydrogels that have been used in conjunction with tissue scaffolding, drug delivery devices, facial cosmetic fillers, wound healing materials, regenerative periodontal therapy, postoperative antimicrobial agents, antibiotic-coated titanium implants, caries preventive agents, orthodontic tooth movement materials, craniofacial defect repair materials, gingival grafts, and pulpal regenerative agents.31,51-54 Likewise, some fibrous hydrogel variants contain animal collagen that could possibly trigger an AG reaction.55
Carrageenan
Red seaweed contains carrageenan, a polysaccharide that possesses a somewhat comparable 1,3 galactosidic bond with AG oligosaccharides.56,57 Carrageenan has been included as a thickening agent in toothpastes, cosmetics, shampoos, and some medications. Use of carrageenan has resulted in dermatopathic lesions, gastrointestinal symptoms, lip angioedema, joint disorders, hepatic disease, and anaphylaxis.57-60 Alginate and agar are seaweed-based impression materials that contain complex polysaccharides that are chemically similar to carrageenan.61 To date, there have not been any documented cases of AGS associated with use of dental alginate. However, in 1992, Rice and colleagues62 reported a cohort of 47 dental students who developed mildly uncomfortable mucocutaneous vesicles within 1 through 2 days after undergoing dental impressions with a commercially available alginate material. Within 2 through 5 days, all lesions had resolved. Although the mechanism of the lesional outbreak was indeterminate, it is somewhat plausible that the observed reactions represented an attenuated AGS-like response to the alginate and its polysaccharide constituent. Accordingly, attending clinicians should carefully consider use of nonalginate and non–algae-based impression materials in patients with AGS.
Casein
Casein phosphopeptide–amorphous calcium phosphate is another bovine derivative that offers the potential to induce an AGS-like event.25 It has been incorporated in topical dental pastes and mouthrinses to enhance enamel remineralization, salivary flow, and caries prevention,63,64 and in a paste that is applied to the fitting side of dentures to improve the salivary flow rate.65 Casein phosphopeptide–amorphous calcium phosphate has also been included in some brands of chewing gum to purportedly stimulate saliva. Although there are apparently no published reports of AGS with products containing casein phosphopeptide–amorphous calcium phosphate, their use in affected patients raises concern about the development of an oral mucosal contact sensitivity reaction or systemic involvement from inadvertent product ingestion. Other dental ingredients that could potentially lead to an AGS reaction include lanolin, which is derived from sheep and has been included as an ingredient in many lip balms. Lactoferrin, an antimicrobial agent derived from bovine milk and human bodily secretions, has been contained in several mouthrinse brands.
Other medication
It is beyond the scope of this article to list all medications that contain AG-like epitopes. More common pharmaceuticals that could potentially precipitate an AG reaction include acetaminophen, antibiotics (ie, penicillin, amoxicillin, doxycycline, and clindamycin), anticoagulant agents (ie, apixaban, enoxaparin, heparin, rivaroxaban, and warfarin), antiplatelet agents (ie, aspirin, clopidogrel, prasurgrel, and ticagrelor), cetuximab, hydroxychloroquine, epoetin alfa, hydromorphone, infliximab, linezolid, lopinavir, milrinone, monoclonal antibodies, propofol, snake antivenom, thrombin, and vasopressin.32,45,46,66-68
Dental products and their excipients that could potentially trigger AGS reactions–identified by DeGenova and Soileau
Adapted from DeGenova MBA A, Soileau K. The Implications of Alpha-Gal Syndrome for Dental and Medical Treatment. New Orleans Dental Association News. 2025;63(6):4.
“These are only a sampling of those medications utilized in patient care in dental settings. The treating dentist should understand the chemistry of all products being utilized in and on a patient diagnosed with AGS:”
-
Topical anesthetic gels
-
Prophy paste
-
Topical fluoride paste
-
Some antiseptic mouthwashes, flosses, and chewing gum products
-
Casein and other milk protein derivatives, such as CPP-ACP, found in some chewing gums, lozenges, mouthwashes, and chairside products marketed to reduce dental erosion, prevent and repair decalcification, and/or desensitize teeth on exposed radicular surfaces
-
Gut sutures
-
Impression material
-
Bone grafts
-
Collagen membranes and other products such as CollaPlug and CollaTape
-
Gelatin-based products, such as pill capsules, Gelfoam, and Surgifoam
-
Glycerin, such as in Propofol and some dentifrices and polishing agents
-
Heparin and thrombin
-
Some vaccines
-
Lidocaine and lidocaine patches (may have a slight amount of animal product, and the AGS safety issue on this anesthetic is controversial among reference sources)
-
Other “veiled” products, including arachidonic acid, arachidyl propionate, lactic acid, lactose monohydrate, stearate and magnesium stearate, stearic acid, myristic acid, oleic acid, and monoclonal antibiotics
-
Acetaminophen-containing oral products, which may contain gelatin, glycerin, lactose monohydrate, magnesium stearate, and stearic acid
-
Aspirin-containing oral products that may contain magnesium stearate
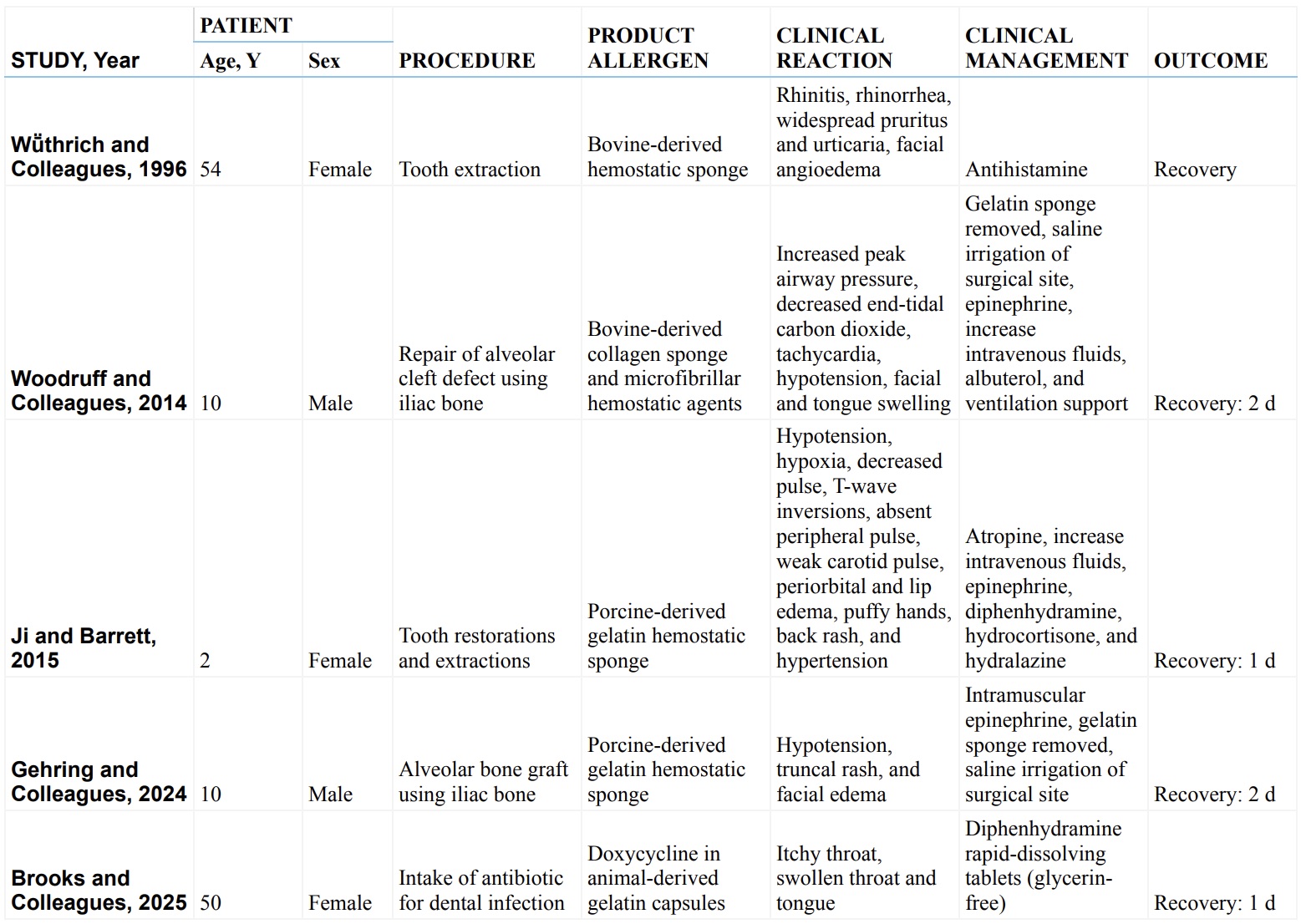
Documented cases associated with alpha-gal reactions from use of dental products.
Adapted from Brooks JK, Hoch NI, Hoch ER, Sultan AS. Alpha-gal syndrome: potential for a hypersensitivity reaction after the use of dental products: Literature review and case report associated with oral health care. The Journal of the American Dental Association. 2025 May 15.
Key publications
Key publications on dentistry and alpha-gal syndrome
- Brooks JK, Hoch NI, Hoch ER, Sultan AS. Alpha-gal syndrome: potential for a hypersensitivity reaction after the use of dental products: Literature review and case report associated with oral health care. The Journal of the American Dental Association. 2025 May 15.
- DeGenova MBA A, Soileau K. The Implications of Alpha-Gal Syndrome for Dental and Medical Treatment. New Orleans Dental Association News. 2025;63(6):4.
Additional publications on dentistry and alpha-gal syndrome
- Wüthrich B, Bianchi-Kusch E, Johansson SG. Allergic urticaria and angioedema caused by a hemostatic sponge of bovine fibrin used in tooth extraction. Allergy. 1996;51(1):49-51.
- Woodruff S, Early R, Qoos W. Anaphylactic reaction with Avitene: a pediatric case report. AANA J. 2014; 82(5):368-374.
- Gehring MB, Freedman JD, Wolfe B, French BM, Khechoyan DY. Intraoperative anaphylaxis to gelatin during alveolar bone grafting for cleft palate. Plast Reconstr Surg Glob Open. 2024;12(3):e5636. doi:10.1097/GOX. 0000000000005636
- Ji J, Barrett EJ. Suspected intraoperative anaphylaxis to gelatin absorbable hemostatic sponge. Anesthesia Progress. 2015 Jan 1;62(1):22-4.
